Potassium Metal Reacts With Silver Chloride
The sodium gas reacts with the potassium chloride to make potassium gas and sodium chloride. It is a liquid under normal conditions.
Page 5 Nitrate Of Silver High Resolution Stock Photography And Images Alamy
Potassium chloride silver nitrate -- potassium nitrate silver chloride.
. 2Na 2Hcl 2NaCl H 2. K AgCl KCl Ag 15. It moves around very quickly on the surface of the water.
A g N O 3 K C l A g C l K N O 3. It makes a pink color when heated in a flame. Potassium metal reacts with silver chloride.
1 1 2 1. They are formed through various chlorination. Potassium nitrate product AgCl.
Shiny or white and atomic number 47. Solutions of tin II nitrate and potassium hydroxide are combined. 1 When dissolved beryllium chloride reacts with silver nitrate dissolved in n water aqueous beryllium nitrate and silver chloride powder are made.
Silver chloride product KCl AgNO3 KNO3 AgCl. Potassium ChlorideSilver Chloride Solution for electrode filling and cloud seeding is generally immediately available in most volumes including bulk quantities. Potassium metal reacts with a solution of silver chloride to create two products at least one of which is a solid 16.
D Potassium metal reacts with water to give potassium hydroxide and hydrogen gas. Potassium chloride reactant AgNO3. 2 H20 - 2 H2g O2g.
The precipitate formed in this reaction is of lead II chloride. This molecule consists of one potassium cation K and one. Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Question 27 Which of the following pairs of compounds will react to form a precipitate. Sodium metal reacts with hydrochloric acid HCl and produces hydrogen gas as one of the products. Solutions of tin II nitrate and potassium hydroxide are combined.
A solution of potassium chloride when mixed with silver nitrate solution forms an insoluble white substance silver chloride. What happens when lead nitrate reacts with potassium chloride. When potassium chloride reacts with silver nitrate a white precipitate of silver chloride is obtained.
Potassium metal reacts with silver chloride. The metals are gas because it is so hot. K is not balanced.
Therefore when the soluble salts silver nitrate and sodium chloride are mixed insoluble silver chloride forms and precipitates out. K AgCl KCl Ag. Chloride compounds can conduct electricity when fused or dissolved in water.
The sodium metal melts and boils to make sodium gas. A mixture of potassium and any of the following compounds produces a weak explosion on impact. It is used to make potassium metal.
2 Na 2 Hcl 2 NaCl H 2. Silver is a chemical element with the symbol Ag from the Latin argentum derived from the Proto-Indo-European h₂erǵ. Almost all alkali metal compounds and nitrates are soluble but most silver compounds are insoluble except for acetates perchlorates chlorates and nitrates.
KCl AgNO3 -- KNO3 AgCl. Aqueous silver nitrate reacts with aqeous potassium iodide in a double-replacement reaction to produce a precipitate of silver iodide. Does potassium chloride and silver nitrate form a precipitate.
Both are white solids. Hydrochloric acid reacts with sodium metal. Place a coefficient of 2 in front of K on the LHS.
The potassium immediately ignites on contact with the acid producing a bright lilac flame that quickly grows until the potassium burns up. This reaction is considered to be a double displacement reaction as lead and potassium. The reaction between Lead Nitrate and potassium chloride leads to the formation of Lead II Chloride and Potassium Nitrate.
It reacts with silver nitrate to make silver chloride. Sodium metal reacts with hydrochloric acid HCl and produces hydrogen gas as one of the products. Aluminum hydroxide sodium nitrate -- aluminum nitrate sodium hydroxide.
Question 16 1 pts Silver nitrate reacts with potassium chloride to produce solid silver chloride and what substance. 2Ag₂Os 4Ags O₂ g. It reacts vigorously with water to form potassium hydroxide a corrosive material and hydrogen a flammable gas.
It reacts with sodium metal when very hot to make potassium metal. Aq H₂g Solid silver oxide decomposes to produce solid silver metal and oxygen gas. Ammonium bromide ammonium iodide cadmium fluoride chromium trifluoride manganous bromide manganous iodide nickel fluoride potassium chlorocuprate silver chloride silver iodide strontium iodide thallous chloride and zinc fluoride Mellor 2.
K s Cl2 g2KCl. It is not reactive. Potassium chloride is heated very hot until it melts.
Lithium metal reacts with liquid bromine. When potassium is added to water what happens. The hydrogen ignites instantly.
Al OH3 3 NaNO3 -- Al NO33 3 NaOH. If the compounds are in aqueous solution then their physical states could be added. Sodium metal is reacted with it.
Write balanced equations for the following word equations. It is used in food processing. Silver nitrate reactant KNO3.
A piece of potassium metal reacts violently when placed in water. When potassium is added to water the metal melts and floats. It is a colorless crystalline solid.
What is the symbol of Argentum. 2Na 2HCl 2NaCl H2 16. The balanced equation is 2K s Cl2 g2KCl s There are two chlorine atoms on the left-hand side LHS and one chlorine atom on the right-hand side RHS.
Add a coefficient of 2 in front of KCl. Aqueous barium chloride reacts with aqueous sodium sulfate to form sodium chloride solution and a precipitate of barium sulfate. Chloride materials can be decomposed by electrolysis to chlorine gas and the metal.
Reaction Worksheet Answer Key. You are given potassium carbonate solid and potassium sulfate. If 346 ml of 0563 M silver nitrate are used with 1484 ml of potassium iodide.
2Li Br2 2LiBr 14. Precipitation reactions and ions in solution Silver nitrate AgNO3 reacts with potassium chloride KCl and a white precipitate is formed. K sp 177 x 10-10 1 AgNO 3.
It is similar to sodium chloride. Formulas for the compounds. Aqueous beryllium chloride aqueous silver nitrate aqueous beryllium nitrate solid silver chloride BeCl 2aq AgNO 3aq BeNO 3 2aq AgCl s 2 When liquid isopropanol C 3 H 8.
The heat from this reaction may be sufficient to ignite the hydrogen. Potassium metal alloys is potassium mixed with some other metal usually sodium. It is an example of a reaction.
KCl aq AgNO3aq KNO3aq AgCl s Answer link. Its molar mass is 7455 gmol. It can be electrolyzed in a water solution to make potassium hydroxide.
Potassium alloy may ignite spontaneously. There are two K atoms on the RHS and only one on the LHS. K AgCl KCl Ag.
The metal is also set. Calcium chloride and silver nitrate sodium iodide and potassium chloride calcium chloride and sodium nitrate Your explanation should be written in complete sentences. O potassium nitrate O potassium nitrite Oxygen O nitrogen O potassium metal Question 17 1 pts The following reaction takes place when an electric current is passed through water.
C Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.

Solved 4 Pts Write Balanced Reactants And Produced Chemical Equation For The Following Chlorine Gas Is Scenarios Flowed Include The State Of The Over Solid Sodium Metal And Reacts To Form

Soluble And Insoluble Salts Solubility Chemistry Silver Chloride
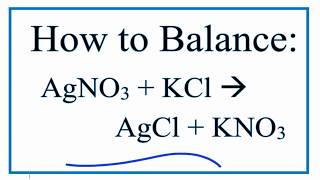
Balance Agno3 Kcl Kno3 Agcl Silver Nitrate And Potassium Chloride Youtube

How To Write The Net Ionic Equation For Agno3 K2cro4 Ag2cro4 Kno3 Youtube

Kate Wolf Soldering Clay Clay Polymer Clay Tutorial Precious Metal Clay

Solved 2 4 Pts Write A Balanced Chemical Equation For The Chegg Com

How To Write The Net Ionic Equation For Agno3 Kcl Agcl Kno3 Youtube

Question Video Determining The Mass Of The Potassium Chloride Analyte When 2g Of Silver Chloride Precipitates Nagwa

14 Potassium Metal Reacts With Silver Chloride K Agcl Kcl Ag 15 Sodium Metal Course Hero

Balance Agno3 Kcl Kno3 Agcl Silver Nitrate And Potassium Chloride Youtube

Solved Predicting Rxn Products Worksheet For Each Item Chegg Com
Potassium Silver Cyanide Kag Cn 2 Pubchem
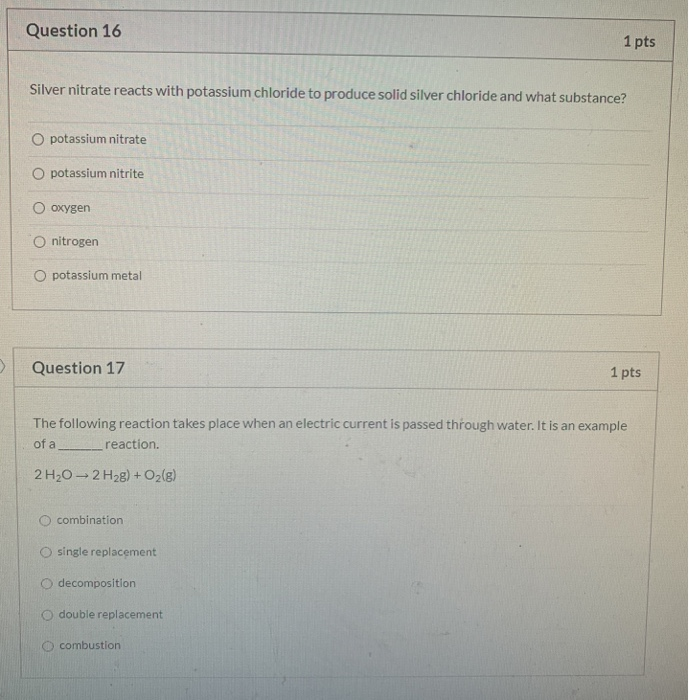
Solved Question 16 1 Pts Silver Nitrate Reacts With Chegg Com
Ppt Igcse Chemistry Powerpoint Presentation Free Download Id 9441135

Sodium Hydroxide Vs Potassium Hydroxide Reaction With Aluminum Be Safe When Using Products With These Chemicals Youtube Sodium Hydroxide Potassium Sodium

Write The Balanced Chemical Equations For The Following Reactions A Calcium Hydroxide Carbon Dioxide Calcium Carbonate Water B Zinc Silver Nitrate Zinc Nitrate Silver C Aluminium Copper Chloride
What Does Silver Nitrate Agno3 Potassium Chloride Kcl Produce With State Symbols Quora

What Happens When Potassium Chloride Reacts With Silver Nitrate

Extracting Iron From Its Ore Chemical Reduction With Carbon Chemistry Iron Ore Blast Furnace
Comments
Post a Comment